Abstract
Background
ALK-positive (ALK+) anaplastic large cell lymphoma (ALCL) is a T-cell lymphoma characterized by a chromosomal translocation involving the ALK gene and expression of ALK protein and CD30. It is a rare lymphoma affecting young people, representing approximately 0.5% of adult lymphomas (Laurent C, JCO 2017). BV-CHP and CHOEP improved outcomes compared to CHOP, with 5-y PFS and OS of 85-90% and 90-97%, respectively (Horwitz SM, ASH 2021; Cederleuf H, BJH 2017; Sibon D, Haematologica 2019; Brink M, Blood 2022). International prognostic index (IPI) has been shown to impact prognosis (Sibon D, Haematologica 2019), however this may not be the best prognostic model. New markers have been retrospectively assessed in children such as histologic subtypes (common vs other), detection of circulating tumor cells by RT-PCR for ALK fusion transcript, and anti-ALK antibody titer, with impact on outcomes (Lamant L, JCO 2011; Mussolin L, Leukemia 2013; Damm-Welk C, Blood 2014; Mussolin L, BJH 2017). Based on these encouraging results, the prospective ALK-OBS trial was initiated to assess the prognostic value of new markers in adults with ALK+ ALCL (ClinicalTrials.gov No. NCT03603847). Here we report the first results of the ALK-OBS trial.
Methods
ALK-OBS is a prospective multicenter observational study conducted by the LYSA. The primary objective was to assess the prognostic value of new markers on PFS and OS of adults with ALK+ ALCL. Secondary objectives included safety, specifically febrile neutropenia and peripheral neuropathy. Key inclusion criteria were as follows: Newly diagnosed ALK+ ALCL, age ≥ 18 years, treatment with 6 cycles of CHOEP-21 according to LYSA guidelines with PET-CT after 3 and 6 cycles of CHOEP. New markers were as follows: 1) Histologic subtypes (common vs other) and ALK staining pattern (cytoplasmic + nuclear vs cytoplasmic; correlation with the ALK partner gene) were determined after central pathology review by 2 expert hematopathologists (LL and CS); 2) Qualitative RT-PCR designed to allow the detection of all ALK fusion transcripts was performed in peripheral blood at baseline (minimal disseminated disease, MDD], after the first cycle of CHOEP (minimal residual disease 1, MRD1) and at the end of treatment (MRDend); and anti-ALK antibody titers were evaluated at baseline and at the end of treatment. Here we report the first results of ALK-OBS study.
Results
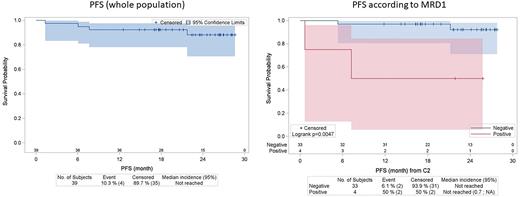
A total of 40 patients (pts) were included between August 2018 and January 2021, of whom 39 received at least one cycle of CHOEP. Median age was 41 years (range 20-78; 6 pts > 60 years), with a male predominance (77%). 30 pts (77%) were stage III-IV, 28 (72%) had PS 0-1, and 24 (62%) had B symptoms. IPI was 0-1, 2, 3 and 4-5 in 13 (33%), 9 (23%), 10 (26%) and 7 (18%) pts, respectively. After central pathology review, histologic subtypes were common (62.5%), small cells (7.5%), lymphohistiocytic (5%), and composite (22.5%). ALK staining was cytoplasmic + nuclear in 59% of cases (predictive of NPM1-ALK fusion). MDD was detectable in 25/38 pts (66%) and anti-ALK antibody titer was ≥ 1/60750 in 10/35 pts (29%). MRD1 was still detectable in 4/37 pts (11%). After a median follow-up of 2 years, there were 4 progression/relapse events and no death, with 2-y PFS and OS of 88% and 100%, respectively. Taking into account the challenge of analyzing small datasets with limited power, the only factor with a significant prognostic impact on PFS was MRD1, with 2-y PFS of 50% vs 92% (p=0.0047) for detectable vs undetectable MRD1, respectively. In all, 36 pts (92%) received the planned 6 cycles of treatment, and 34 pts (87%) received all cycles of etoposide. There were 3 permanent treatment discontinuations (1 for progression after cycle 2, and 2 for adverse event after cycles 4 and 5, respectively). There were 13 febrile neutropenia episodes for 227 cycles administered (6%), and 9 pts experienced peripheral sensory neuropathy (7 G1, 1 G2 and 1 G3), 8 of which completely resolved (G2 remained stable).
Conclusions
ALK-OBS is the first prospective study dedicated to adult ALK+ ALCL. MRD1 identified a high-risk group, for which specific approaches may be needed. CHOEP was overall well tolerated and induced high rates of PFS and OS.
Disclosures
Sibon:AbbVie: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Ysebaert:Abbvie, Astra-Zeneca, Janssen, Roche, Beigene, BMS/Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Thieblemont:Bristol Myers Squibb: Consultancy, Honoraria, Other: Travel Support; Roche: Consultancy, Honoraria, Other: Travel Support; AbbVie: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Other: Travel Support, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel Support; Incyte: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Other: Travel Support; Celgene: Consultancy, Honoraria, Other: Travel Support. Bouabdallah:Kite/Gilead: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Dupuis:abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees. Bachy:Hospices Civils de Lyon: Current Employment; Amgen, BMS: Research Funding; Kite, Gilead, Novartis, Roche, Incyte, Miltenyi Biotech, Takeda, Sanofi: Honoraria; Roche, Gilead, ADC Therapeutics, Takeda, Novartis, Incyte: Membership on an entity's Board of Directors or advisory committees. Sylvain:Novartis: Consultancy, Honoraria; gilead: Consultancy, Honoraria. Feugier:AstraZeneca, Janssen, Abbvie, Beigene, Gilead: Membership on an entity's Board of Directors or advisory committees, Other: Congress Invitations. Le Calloch:AbbVie, Gilead, Janssen, Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal